We help trial teams stay organized, compliant, and patient-focused every step of the way.
With over 10 years of experience supporting 100+ clinical trials and collaborating with 50+ sponsors and research sites, OnePath Research Services delivers audit-ready documentation, streamlined workflows, and compassionate participant resources — all designed to advance your clinical research journey.
Clinical Trial Sites
Expert support for smooth, compliant trial setups.
Sponsors
Help maintain audit-ready documentation.
Participants
Helping you feel informed, prepared, and supported.
About OnePath Research Services
OnePath Research Services is a specialized consulting firm providing documentation, compliance, and operations support to organizations involved in clinical research and regulated industries. We help CROs, sponsors, and small research-focused businesses streamline their workflows, strengthen audit readiness, and maintain regulatory alignment through high-quality documentation and support tools.
Our services include SOP creation, site and study-level trackers, slide deck formatting, regulatory document reviews, and document automation strategies. We work with precision and discretion—delivering polished, audit-ready materials designed to support operational excellence.
In addition to our professional services, OnePath proudly offers free support services for clinical trial participants and patients.
We help individuals understand consent forms, identify relevant clinical trials, and serve as a compassionate resource—offering guidance, clarity, and emotional support throughout their research journey.

Our Core Services
Specialized consulting and resources designed to streamline operations, ensure compliance, and support every clinical stakeholder.

Regulatory & Compliance Consulting
We help clinical trial sites and sponsors maintain audit-ready documentation through customized regulatory consulting. From TMF quality checks to SOP development and site startup packages, our services ensure your team is compliant, efficient, and prepared for inspection.
Explore More
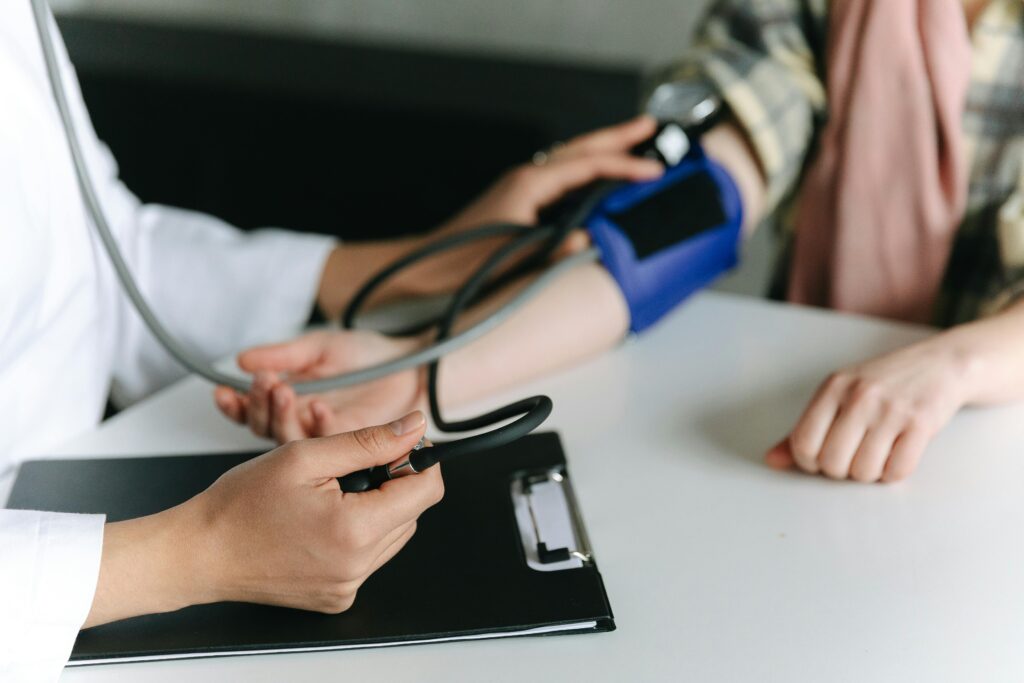
Trial Participant Support Hub
We provide tools and resources to help patients understand clinical trials and feel supported every step of the way. From preparing for informed consent to navigating transportation or follow-up visits, we make participation more accessible, inclusive, and compassionate.
Explore More

Digital Template & Resource Store
Our ready-to-use regulatory templates are designed for clinical researchers, coordinators, and startup sites looking to streamline operations. From CV formats and DOA logs to TMF QC checklists and SOPs, each tool is professionally formatted and trial-site ready.
Explore More
Our Mission
To support research-driven organizations with documentation and operational tools that ensure compliance, improve efficiency, and elevate professional presentation—while empowering clinical trial participants through compassionate, patient-centered support.

Our Values
Accuracy
We prioritize detail, clarity, and compliance in every deliverable.
Professionalism
Our service reflects the high standards of the industry we support.
Confidentiality
We operate with discretion and care, protecting sensitive data and proprietary information.
Dependability
Clients count on us for consistent, timely, and high-quality execution.
Service
We view our role as a partner, not just a provider—here to help research move forward.
Compassion
We are committed to supporting the real people behind every trial, treating patients and participants with empathy and respect.

Our Goals
- Deliver consistent, audit-ready documentation and operational tools to research professionals and sponsors
- Become a trusted back-office partner for clinical teams and CROs seeking streamlined documentation and compliance support
- Grow a vetted network of expert consultants to expand capacity while maintaining regulatory quality standards
- Provide meaningful, no-cost assistance to patients and participants navigating the clinical trial landscape
- Build a sustainable business that supports innovation, excellence, and compassion in clinical research

0
+
Clinical Trials Supported
0
+
Sponsor & CRO Partnerships
0
+
Documents Delivered
0
+
Participants Assisted
What Our Clients Say
At OnePath Research Services, we pride ourselves on delivering dependable, high-quality support that makes a difference. Here’s what our clients and partners have to say about working with us.
![]()
OnePath’s regulatory consulting transformed our site’s documentation process. Their templates and audits made inspections stress-free.
![]()

✯✯✯✯✯
Operations Manager
Cosmetics Manufacturer
![]()
The patient support tools helped our participants feel informed and cared for throughout the trial. Truly compassionate service.
![]()

✯✯✯✯✯
R&D Director
Pharmaceutical Company
![]()
Their SOP customization and TMF QC checklists saved us weeks of administrative work. Highly recommend for any CRO.
![]()

✯✯✯✯✯
Production Supervisor
Food Processing Plant
![]()
OnePath is a reliable partner who understands both compliance and the patient perspective. Their support is invaluable.
![]()

✯✯✯✯✯
Procurement Manager
Chemical Manufacturer
![]()
OnePath’s regulatory consulting transformed our site’s documentation process. Their templates and audits made inspections stress-free.
![]()

✯✯✯✯✯
Operations Manager
Cosmetics Manufacturer
![]()
The patient support tools helped our participants feel informed and cared for throughout the trial. Truly compassionate service.
![]()

✯✯✯✯✯
R&D Director
Pharmaceutical Company
![]()
Their SOP customization and TMF QC checklists saved us weeks of administrative work. Highly recommend for any CRO.
![]()

✯✯✯✯✯
Production Supervisor
Food Processing Plant
![]()
OnePath is a reliable partner who understands both compliance and the patient perspective. Their support is invaluable.
![]()

✯✯✯✯✯
Procurement Manager
Chemical Manufacturer
Request a no-cost site
assessment with OnePath.
Ready to streamline your research operations or support your clinical trial participants with confidence? Connect with OnePath Research Services for expert guidance, audit-ready tools, and compassionate support tailored to your needs.
Request a Free Assessment
